#SIP/#ASTRO 3D: #Neutron #Stars Contribute Little, but Something's Making #Gold
ISSUED BY SCIENCE IN PUBLIC IN SPOTSWOOD, VICTORIA, AUSTRALIA, ON BEHALF OF ASTRO 3D, THE ARC CENTRE OF EXCELLENCE FOR ALL-SKY ASTROPHYSICS IN 3 DIMENSIONS,
ELEMENTS OF SURPRISE: NEUTRON STARS CONTRIBUTE
LITTLE, BUT SOMETHING’S MAKING GOLD, RESEARCH FINDS
** Colliding neutron stars were touted as the main source of some of the heaviest elements in the periodic table. Now, not so much. **
Neutron star collisions do not create the quantity of chemical elements previously assumed, a new analysis of galaxy evolution finds. The research also reveals that current models can’t explain the amount of gold in the cosmos -- creating an astronomical mystery.
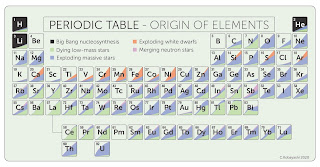
The work has produced a new-look periodic table, showing the stellar origins of naturally occurring elements from carbon to uranium.
All the hydrogen in the universe -- including every molecule of it on Earth -- was created by the Big Bang, which also produced a lot of helium and lithium, but not much else. The rest of the naturally occurring elements are made by different nuclear processes happening inside stars. Mass governs exactly which elements are forged, but they are all released into galaxies in each star’s final moments -- explosively in the case of really big ones, or as dense outflows, similar to solar wind, for ones in the same class as the Sun.
“We can think of stars as giant pressure cookers where new elements are created,” explained co-author Associate Professor Karakas, from Australia’s ARC Centre of Excellence for All Sky Astrophysics in 3 Dimensions (ASTRO 3D). “The reactions that make these elements also provide the energy that keeps stars shining bright for billions of years. As stars age, they produce heavier and heavier elements as their insides heat up.”
Half of all the elements that are heavier than iron -- such as thorium and uranium -- were thought to be made when neutron stars, the superdense remains of burnt-out suns, crashed into one another. Long theorised, neutron star collisions were not confirmed until 2017. Now, however, fresh analysis by Karakas and fellow astronomers Chiaki Kobayashi and Maria Lugaro reveals that the role of neutron stars may have been considerably overestimated -- and that another stellar process altogether is responsible for making most of the heavy elements.
“Neutron star mergers did not produce enough heavy elements in the early life of the universe, and they still don’t now, 14 billion years later,” said Karakas. “The universe didn’t make them fast enough to account for their presence in very ancient stars, and, overall, there are simply not enough collisions going on to account for the abundance of these elements around today.”
Instead, the researchers found that heavy elements needed to be created by an entirely different sort of stellar phenomenon -- unusual supernovae that collapse while spinning very fast and generating strong magnetic fields.
The finding is one of several to emerge from their research, which has just been published in The Astrophysical Journal. Their study is the first time that the stellar origins of all naturally occurring elements from carbon to uranium have been calculated from first principles.
The new modelling, the researchers say, will substantially change the presently accepted model of how the universe evolved.
“For example, we built this new model to explain all elements at once, and found enough silver but not enough gold,” said co-author Associate Professor Kobayashi, from the University of Hertfordshire in the UK. “Silver is over-produced but gold is under-produced in the model compared with observations. This means that we might need to identify a new type of stellar explosion or nuclear reaction.”
The study refines previous studies that calculate the relative roles of star mass, age and arrangement in the production of elements. For instance, the researchers established that stars smaller than about eight times the mass of the Sun produce carbon, nitrogen, and fluorine, as well as half of all the elements heavier than iron.
Massive stars over about eight times the Sun’s mass that also explode as supernovae at the end of their lives, produce many of the elements from carbon through to iron, including most of the oxygen and calcium needed for life.
“Apart from hydrogen, there is no single element that can be formed only by one type of star,” explained Kobayashi. “Half of carbon is produced from dying low-mass stars, but the other half comes from supernovae. And half the iron comes from normal supernovae of massive stars, but the other half needs another form, known as Type Ia supernovae. These are produced in binary systems of low mass stars.”
Pairs of massive stars bound by gravity, in contrast, can transform into neutron stars. When these smash into each other, the impact produces some of the heaviest elements found in nature, including gold.
On the new modelling, however, the numbers simply don’t add up.
“Even the most optimistic estimates of neutron star collision frequency simply can’t account for the sheer abundance of these elements in the Universe,” said Karakas. “This was a surprise. It looks like spinning supernovae with strong magnetic fields are the real source of most of these elements.”
Co-author Dr. Maria Lugaro, who holds positions at Hungary’s Konkoly Observatory and Australia’s Monash University, thinks the mystery of the missing gold may be solved quite soon.
“New discoveries are to be expected from nuclear facilities around the world, including Europe, the USA and Japan, currently targeting rare nuclei associated with neutron star mergers,” she said. “The properties of these nuclei are unknown, but they heavily control the production of the heavy element abundances. The astrophysical problem of the missing gold may indeed be solved by a nuclear physics experiment.”
The researchers concede that future research might find that neutron star collisions are more frequent than the evidence so far suggests, in which case their contribution to the elements that make up everything from mobile phone screens to the fuel for nuclear reactors might be revised upwards again.
For the moment, however, they appear to deliver much less buck for their bangs.
Commenti
Posta un commento